- Recombinant Human Serum Albumin
- Recombinant Growth factors
-
Proteins for Cosmetics Ingredients
Recombinant Human basic Fibroblast Growth Factor (OsrhbFGF) Recombinant Human Epidermal Growth Factor (rhEGF) Recombinant Human Vascular Endothelial Growth Factor (OsrhVEGF) Recombinant Human Keratinocyte Growth Factor (OsrhKGF) Recombinant Human Lactoferrin (OsrhLF) Recombinant Human Fibronectin (OsrhFN) Recombinant Human Lysozyme (OsrhLYZ)
- Other Recombinant Proteins
OsrHSA Premium (Recombinant Human Serum Albumin)
Human serum albumin (HSA) is the most well studied plasma protein and an attractive macromolecular carrier due to its availability in pure form and its biodegradability, nontoxicity and non-immunogenicity. It has a serum half-life of approximately 20 days, and is widely used as a stabilizing component in pharmaceutical and biologic products, such as vaccines, cell and gene therapies and coatings for medical devices. Due to the potential existence of various infectious disease pathogens in the human blood, especially AIDS, hepatitis B and other viruses, these virus may be brought with HSA to the final products.
OsrHSA Premium, a recombinant human serum albumin derived from rice grains, can provide an excellent solution to utilise a safer, more defined and consistent material. Compared to plasma human serum albumin (pHSA), OsrHSA Premium has the same second and tertiary structure, physiological and biochemical properties with pHSA, while higher purity and excellent batch consistency.

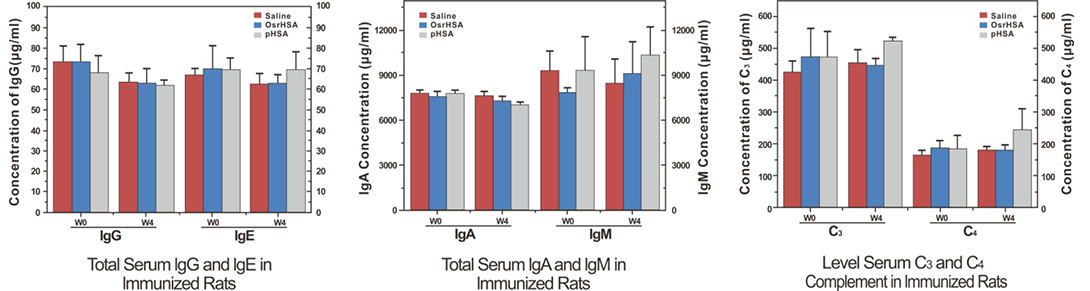
The titers of individual immunoglobulins IgG, IgM, IgE, and IgA showed no significant difference among the pHSA, OsrHSA, and saline groups, and the titers of C4 complement were not significantly different.
The OsrHSA Premium has officially obtained the Drug Master Files (DMF) number(No. 029648) from US Food and Drug Administration (FDA) in 2015, which showed that the OsrHSA Premium has good safety and the rice expression platform has been highly approved by FDA and the international market. The rice expression platform is a world-leading technology and produces cost-effective OsrHSA Premium that is safe and can help to satisfy an increasing worldwide demand for human serum albumin, thus open new doors in its applications and contribute to self-sufficiency in blood and blood products.



